Suppression of the Mast Cell-Dependent Weal and are Response In Vivo by Glucocorticoids in Human Skin
Hari Prasad Sonwani
Department of Pharmacy, Apollo Group of Institution, Durg, India
Published Date: 2023-12-12DOI10.36648/ipsdsc.8.4.99
Hari Prasad Sonwani*
Department of Pharmacy, Apollo Group of Institution, Durg, India
- *Corresponding Author:
- Hari Prasad Sonwani
Department of Pharmacy,
Apollo Group of Institution, Durg,
India,
E-mail: harisonwani10@gmail.com
Received date: November 11, 2023, Manuscript No. IPSDSC-23-18117; Editor assigned date: November 14, 2023, PreQC No. IPSDSC-23-18117 (PQ); Reviewed date: November 28, 2023, QC No. IPSDSC-23-18117; Revised date: December 05, 2023, Manuscript No. IPSDSC-23-18117 (R); Published date: December 12, 2023, DOI: 10.36648/ipsdsc.8.4.99
Citation: Sonwani HP (2023) Suppression of the Mast Cell-Dependent Weal and are Response In Vivo by Glucocorticoids in Human Skin. Skin Dis Skin Care Vol.8 No.4: 99.
Abstract
This study looks at the relative roles that glucocorticoids play in inhibiting the weal and are in human skin. These roles include lowering the responsiveness of the vasculature to histamine, reducing mast cell degranulation and reducing mast cell recruitment and maturation. Healthy human volunteers had one forearm treated with 0.05% clobetasol propionate for 24 hrs (n=6) or every day for 21 days (n=10). The control arm was the other one. Intradermal injections of 20 milliliters of 0.3 millimeter codeine produced both weal and are reactions. Doppler imaging with a scanning laser was used to measure the regions of the responses. Histamine release was evaluated using micro dialysis. In 4 mm punch biopsies, mast cell counts and tissue histamine levels were measured. Utilizing histamine (20 ml of 1 mMi.d.), the vasculature's condition was evaluated. At 24, no significant effects were observed. Clobetasol decreased the areas of the codeine-induced weal and are responses by 58% and 59%, respectively, at 21 days (both P=0.006). The total tissue histamine concentration decreased by 52% (P=0.006) and the number of mast cells decreased by 47% (P=0.014). The release of histamine caused by codeine was decreased by 44% (P=0.022). Histamine-induced weal was significantly decreased (P=0.019), but not they are. A 15% thinning of the skin by clobetasol was discovered by echography. These findings show that, rather than corticosteroids inhibiting mast cell degranulation, decreased mast cell counts and tissue histamine content are the main causes of the decreased weal and are reactions to codeine after clobetasol administration.
Keywords
Glucocorticoids; Clobetasol; Mast cell; Weal; Flare; Skin; Histamine; Human
Abbreviations
AEC: Aminoethylcarbazole; SLDI: Scanning Laser Doppler Imaging.
Introduction
Although glucocorticoids have a well-established therapeutic impact in lowering allergic inflammation and slowing the late phase of the allergic response, it is less known how beneficial they may be for the mast cell-mediated early phase of the allergic response. For example, they may be beneficial when taken over longer periods of time but are not very helpful when applied immediately to urticaria. However, physicians must weigh the advantages of treatment against the possible longterm negative effects on the skin, such as thinning and a decline in cutaneous immune defenses, when thinking about using glucocorticoids for extended periods of time. Glucocorticoids have the potential to decrease the mast cell-dependent weal and flare response in the skin through three different pathways. The initial is a decrease in the vasculature's reactivity to mast cell mediators, a second effect on mast cell maturation and recruitment, and an inhibitory influence on the release of mast cell mediators. It is doubtful that mast cells will suppress the release of mediators. Studies conducted in vitro have shown that incubating human mast cells originating from the lung, gut, or skin for 24 hrs with the powerful synthetic glucocorticoid dexamethasone did not prevent the release of mediators from these cells [1,2]. Additionally, when glucocorticoids are administered in vivo for 48 hrs or shorter, the early phase allergy responses do not become less severe, suggesting that mast cell degranulation is not prevented [3-6]. Steroids may prevent the recruitment and maturation of mast cells within tissues, as evidenced by the reduction in the size of weal and flare responses triggered in the skin by allergens or codeine when glucocorticoids are administered for longer than a week [7,8]. Then, direct evidence supporting this theory was acquired from biopsy tests, which demonstrated a drop in tissue histamine levels and mast cell numbers starting in week 3 of glucocorticoid treatment for the skin [8,9]. This was verified by an electron microscopy investigation that depicted the mast cells in steroids by Lavker and Schechter [9]. Considered skin to be "degenerating" and "dying." This observed effect is most likely the result of both an increased rate of mast cell death among the existing mast cells and a decrease in mast cell recruitment to the tissue. Glucocorticoids' capacity to cause vasoconstriction suggests that they alter the response of the vasculature to mast cell mediators is not clear-cut; some researchers [7,10-13] reported no effect, while others [5,14,15] reported no effect. In order to explore the mechanisms by which glucocorticoids block the mast cell-dependent weal and flare response in human skin in vivo, we have contrasted the effects of clobetasol propionate administered topically for 24 hrs with administration once a day for three weeks. Weal and flare areas were measured using scanning laser Doppler imaging; histamine release was evaluated by micro dialysis; and total tissue and mast cell counts were determined through biopsies. Histamine levels and skin thickness monitoring with echography.
Materials and Methods
Examine the population
Ten male participants, ranging in age from 19 to 35 (mean 23.3 ± 1.12), provided written informed consent and agreed to take part in the research. The South West and Southampton Joint Research Ethical Committee provided ethical approval (application no.08/99). Subjects using corticosteroids, nonsteroidal anti-inflammatory medications, antihistaminic medications, or medications that impact the cardiovascular system were all excluded. Additionally disqualified were participants with dermato-logical or cardiovascular disorders.
Deumal puovocation using codeine and histamine
Mast cell degranulation was induced by intradermal injections of 20 μl of 0.3 mM codeine (Martindale Pharmaceuticals, Romford, Essex, U.H.) in Phosphate Buffered Saline (PBS). As an affirmative reference, 20 μl of 1 μM Histamine (Powelle, Sigma and U.H.) was injected. Both shots were administered. Using a U-100 insulin syringe and a 27 gauge needle, place the needle 1 mm from and parallel to the micro dialysis iber (Myjector, Terumo Europe NV, Leuven, Belgium).
Evaluation of flaue and weal responses
As previously mentioned [16], changes in cutaneous blood lux were measured using scanning laser Doppler imaging (SLDI, Moor LDI, Moor Instruments Ltd, Axminster, Devon, U.H.). A 5 cm square skin area was scanned to create laser Doppler pictures, which provided around 100,000 data points for processing. Using the manufacturer's so tware, the regions of the weal and lare reactions were computed from the saved photos. Our experience has demonstrated that weal and lare area variations can be precisely quanti ied. Variations in perfusion to ± 5% and ± 0.05 cm2 [16].
Evaluation of histamine released in living things
Each participant received two linear cutaneous micro dialysis ibers (Gambro model GFE 18, Gambro Dialysaten AG, Germany) with a diameter of 21 ± μm and a molecular mass cutoff of 2 kDa. Under topical local anesthesia (EMLA cream; 2.5%, prilocaine, 2.5% lignocaine, Astra AB, Sweden), they were inserted 5 cm apart for a length of 20 mm at a depth of ~0.α-0.8 mm into the volar area of the forearm, as previously described [17]. Following a two-hour recovery time from trauma and local anesthesia, sterile phosphate buffered 0.9% NaCl saltwater was perfused into the probes at utilizing a micro infusion pump, 5 μl min-1 μl min (Micro dialysis/CMA/100, Biotech, Luton, U.H.). With dialysate, gathered every two minutes between the time of the injection (baseline samples) and 20 mins a ter it and kept at -20°C before the histamine spectrofluorometric test [18].
Histamine content and dermal mast cell number determination after micro dialysis, a 4 mm punch biopsy was obtained and a single site in each forearm's treated area was injected with 2% xylocaine/adrenaline. After cutting each specimen in half, each half underwent the following procedures. The detection of mast cell nuclei after being fixed in 10% buffered formaldehyde; one half of the biopsy was processed into sections 5 μm thick were cut from prana. Next, the slices underwent immunocytochemical staining for human using antibody AA1 to tryptase mast cells [19]. Amineethylcarbazole (AEC) solution was used to visualize tryptase and Mayers hematoxylin was used as a counterstain. The number of nucleated cells in the entire slice that were tryptase-positive was counted in a single blind manner by a single observer. Using a computerized image analysis system (Color vision 1.7 α by Improvision, Coventry, U.H.), the area of intact dermis in each sample was measured, along with the number of mast cells expressed per millimeter. Histamine determination in tissue to measure the remaining portion of the 4 mm punch biopsy, 200 μl of distilled water was added to a preweighed polypropylene tube. The tube was then weighed once more weigh the biopsy and freeze it at a temperature of -80°C. Only the biopsies were frozen at -20°C and thawed six times before the histamine assay to disturb histamine is released from the undamaged cells. After that, histamine was quantified using immunoassay (Immunotech, France). Next, histamine was measured in milligrams per tissue.
Thickness of skin detection
Using echography, the combined thickness of the epidermis and dermis was assessed (DermaScan C version 3, Cortex Technology, Denmark). Within the treated portions of each forearm, two independent scans, each measuring 2 cm2, were performed. Three measurements of skin thickness were obtained from each scan and the mean of the six measurements was used to calculate an estimate of skin thickness in millimeters.
Examine protocols
The subjects made two visits to the laboratory. Both forearms' skin thicknesses were measured on the initial appointment. After that, the subjects received 0.05% propionate clobetasol ointment (Dermovate, Glaxo, Middlesex, U.H.). Base control (white soft paraAn, Adams Healthcare, Leeds, U.H.) and instructed to smear a 1 cm ointment length into an area measuring roughly 10 × 5 cm on each forearm. This application could be done once every 24 hrs for 21 days, or every day before returning to the laboratory. During the second visit to both experiments, intradermal injections of codeine and histamine were administered along with the insertion of two micro-dialysis fibers into each forearm. Every 2 mins for 4 mins prior to and 20 mins following injection, dialysate was collected. To estimate the weal and flare areas, laser Doppler images were collected prior to injection and at 5,10,15, and 20 mins following injection. Only the data for 10 mins are included in this article because estimations of weal and flare regions were qualitatively identical at all-time points. The part on outcomes. A biopsy was collected from each forearm during the 21-days study on a healthy skin area that had been treated with the ointments. Measurements of skin thickness were repeated. After reweighing the ointment tubes, it was determined that the average daily doses of clobetasol propionate and white soft paraan were 307 ± 20 and 432 ± 44 mg, respectively.
Figures
The study's data were not normally distributed, so the results are presented as medians with 95% confidence intervals. The Wilcoxon Signed Rank Test was used to analyze differences between the basic control site and the clobetasol-treated sites. P<0.05 was considered the statistical significance threshold.
Results
Neal's reactions
In the experiment where clobetasol was administered for just 24 hrs, there were no weal responses were statistically significantly inhibited. After administering codeine, the weal areas in the clobetasol and control locations were 0.α0 (0.33 −0.84) cm2 (median with 95% confidence limits) and 0.89 (0.52-1.40) cm2 (P=0.059, n=α), in that order. In response to histamine, the equivalent areas of the weals were 0.53 (0.3 α-0.7 α) cm2 and 0.47 (0.39-0.7 α) cm2 (P=1.00, n=α).
Flare reactions
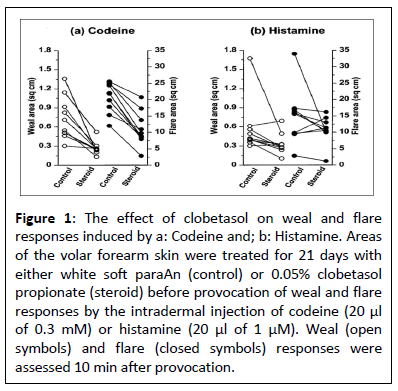
There were no statistically significant suppressions of flare reactions over the 24-hour investigation. Following codeine injection, the flare areas in the clobetasol and control locations were 20.8 (1 α.2-4.8) cm2 and 24.2 (18.0−29.0) cm2 (P=0.402, n=α) correspondingly. In reaction to histamine, the equivalent areas of the flares were 1α.7 (11.7-25.7) cm2 and 23.9 (13.8-30. α) cm2 (P=0.295, n=α) clobetasol decreased codeine-induced flare reactions during the 3-week trial (Figure 1, the median. The flare area at 10 minutes was 9.3 (8.2-4.7) cm2 in the clobetasol-treated arm and 21.9 (17. α-24. α) cm2 in the control arm; this is a 58% reduction (P<0.00 α, n=10). After receiving histamine injection, the corresponding results were 10.8 (7.9-13.3) cm2 as opposed to 15.7 (9.5-21.7) cm2, in that order. P=0.12 α indicates that the reported 31% reduction was not statistically significant.
Figure 1: The effect of clobetasol on weal and flare responses induced by a: Codeine and; b: Histamine. Areas of the volar forearm skin were treated for 21 days with either white soft paraAn (control) or 0.05% clobetasol propionate (steroid) before provocation of weal and flare responses by the intradermal injection of codeine (20 μl of 0.3 mM) or histamine (20 μl of 1 μM). Weal (open symbols) and flare (closed symbols) responses were assessed 10 min after provocation.
Induced histamine release by cocaine
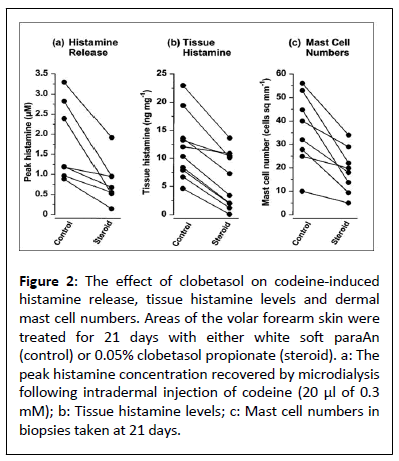
Using micro dialysis, histamine that had been released into the dermis after a codeine injection was found. In all the 24-hour and 3-week investigations, there were no variations in the histamine release time course between the control and clobetasol propionate treated sites, with peak releases happening at 2-4 min. The 24-hour trial found that the median concentrations for clobetasol therapy did not significantly decrease codeine-induced histamine release. 1.74 for both the steroid-treated and control locations 1.41 (0.70-3. α 5) μM and 0.72-7.14 μM (P=0.059). Within the peak histamine levels (Figure 2 decreased after a 3-week study. With clobetasol therapy, from control levels of 1.20 (0.95-2.5 α) μM (44%reduction, P=0.022, n=7) to a median concentration of 0.α7 (0.34-1.43) μM.
Number of dermal mast cells and histamine concentration
The dermal mast cells, which mostly seemed to be gathered around the blood vessels, were reduced at the clobetasol treatment location after three weeks, 18.9 (11.5-α .9) cells mm2 as opposed to 3 α.0 (24.8-48.9) cells mm2 in the control arm (Figure 2). A statistically significant 47% decrease in mast cell counts was observed (P<0.014, n=8). The median in the same biopsies. At the site treated with clobetasol, the tissue histamine concentration was 5.37 (1.98-10.44) ng histamine mg-1 of tissue, whereas at the control site, it was 11.20 (7.90-1α.33) ng histamine mg-1 of tissue, indicating a 52% (P=0.00 α, n=10) reduction (Figure 2).
Figure 2: The effect of clobetasol on codeine-induced histamine release, tissue histamine levels and dermal mast cell numbers. Areas of the volar forearm skin were treated for 21 days with either white soft paraAn (control) or 0.05% clobetasol propionate (steroid). a: The peak histamine concentration recovered by microdialysis following intradermal injection of codeine (20 μl of 0.3 mM); b: Tissue histamine levels; c: Mast cell numbers in biopsies taken at 21 days.
Skin thinning
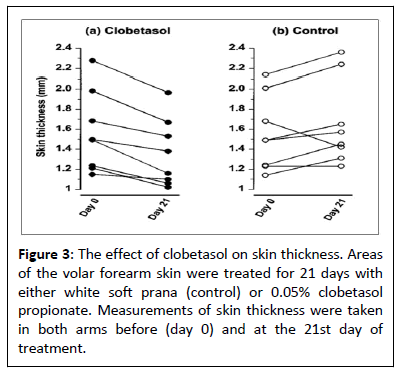
After three weeks of clobetasol treatment, the total dermal and epidermal thickness decreased by 15% (Figure 3), from 1.49 (1.22-1.89) mm to 1.27 (1.08-1.70) mm (P=0.014, n=8). The skin thickness in the base-treated arm did not significantly alter; at the start and finish of treatment, the values were 1.49 (1.24-1.85) mm and 1.51 (1.34-1.9 α) mm, respectively (P=0.272, n=8).
Discussion
These current investigations show that a single application on weal and flare responses brought on by either codeine or histamine, clobetasol administered 24 hrs before to the test had no discernible effect. However, after three weeks of clobetasol treatment, there was a noticeable decrease in codeine reactions and a smaller inhibition to those induced by histamine. The major mechanism underlying this reduction is a reduction in the number of dermal mast cells present within the skin with a consequential reduction in total histamine. A reduction of the responsiveness of the vasculature to histamine appears to be a minor contributory factor.
Clobetasol's impact on skin thickness is shown in Figure 3. For a period of 21 days, 0.05% clobetasol propionate or white soft prana (control) were applied to specific areas of the volar forearm skin. Skin thickness measurements were made in both arms both on day 0 and day 21 of the treatment. Blanching can be seen with the naked eye and through colorimetry; nevertheless, scanning laser Doppler imaging, with the best configuration for identifying increases in epidermal blood flow, was unable to detect it [20]. The response to histamine remained unaltered, indicating that clobetasol did not alter the vasculature's capacity to respond to vasodilator stimuli at this particular moment. Furthermore, the observations that histamine release and the weal and flare response were not suppressed support that steroid degranulation of mast cells was not inhibited [1,2]. According to earlier studies [7,8], the glucocorticoid can decrease the amount of weal and flare reaction when given topically for three weeks. We employed micro dialysis and biopsies as two methods to look into the mast cell dependent mechanisms of this reduction. The histamine release time course in the micro dialysis research matched that previously reported, both with and without glucocorticoid administration [21]. But as compared to the control group, the median peak histamine levels in the clobetasol-treated arm was 44% lower. Dermal mast cell count was 47% lower in the biopsy research, and the tissue histamine was 52% lower in skin treated with clobetasol than with control skin. The median weal and flare areas were correspondingly reduced by 59% and 59%, respectively. When combined, these findings imply that one of the main factors preventing the weal and flare response is a decrease in the quantity of histamine generated in reaction to codeine. Furthermore, rather than a result of degranulation inhibition, the decreased histamine release is a result of fewer mast cells. It is consistent with earlier findings that glucocorticoids do not prevent human mast cell degranulation [1,2,8,9]. Nevertheless, the majority of mechanistic research has been done on rodent mast cells. Glucocorticoids clearly suppress the release of mediators [22-24]. The most possible reason for this discrepancy is because humans, along with other primates and guinea pigs, are "cortisone resistant," but rats and mice are "cortisone sensitive" animals [25].
Previous research has been confirmed by the decrease in mast cell counts and histamine levels in the skin [8,9]. There are three potential explanations for this decrease in mast cell numbers and tissue histamine content: A decrease in the recruitment of mast cell precursors, a decrease in dermal factors necessary for mast cell maturation and histamine synthesis, or an increase in apoptosis. Of them, inhibiting histidine decarboxylase activity would result in a decrease in histamine synthesis [26]. Appear improbable given that the measured histamine level per mast cell in biopsies from steroid-treated and control skin was comparable, at 2.84 and 3.11 pg cell-1, respectively. The decrease in local mast cell growth factor synthesis caused by glucocorticoids is a more plausible explanation. Stem cell factor is one potential factor. It is a cytokine that is essential for mast cell development, recruitment, and survival [27-30], and glucocorticoids of [31] inhibit NF-kB-stimulated transcription by dermal fibroblasts. Another candidate is IL-4, which has been demonstrated to promote apoptosis of cultured murine mast cells upon depletion by glucocorticoids [32].
Taking into account how steroids affect the vasculature, the direct and indirect effects of mast cell mediators must be distinguished. Histamine directly causes an initial vasodilatation at the injection site, which is followed by the formation of a weal. According to Moy et al., [33] the latter is the direct consequence of histamine-induced endothelial cell contraction, which permits the exudation of plasma proteins into the extravascular space. This process may be inhibited by steroids [11,34]. Nonetheless, there is proof that after α-10 days of continuous treatment, tolerance to these steroid effects develops [12,35]. At 21 days, our data indicated a slight but statistically significant inhibitory impact of clobetasol.
As opposed to the weal, the histamine-induced flare is an indirect consequence of the drug; neuropeptides released during histamine-stimulated axon reflexes influence the broader vasodilator response [21]. Clobetasol's inability to produce a statistically significant suppression of the histamine-induced flare response suggests that glucocorticoids had no influence on the histamine-induced activation of sensory nerves or the vasculature's reactivity to vasodilator neuropeptides in this investigation.
A 15% decrease in total dermal and epidermal thickness was seen after 21 days of daily use of the strong topical glucocorticoid clobetasol. Informal assessments conducted on the individuals two to three weeks later revealed that the abrupt thinning was reversed when the treatment was stopped. These findings support earlier research that indicates a significant thinning. Of the skin with even a brief course of corticosteroid therapy [36,37] and highlight the caution that must be taken when administering strong corticosteroids in dermatology. Based on these investigations, the main way that long-term glucocorticoid medication inhibits human weal and flare is by decreasing the amount of mast cells epidermal cells, even if they have a contributing component that inhibits the vasculature's reactivity [38].
Conclusion
These results demonstrate that reduction of the weal and flare responses to codeine following clobetasol treatment, results primarily from reduced mast cell numbers and tissue histamine content rather than inhibition by corticosteroids of mast cell degranulation. These findings show that, rather than corticosteroids inhibiting mast cell degranulation, decreased mast cell counts and tissue histamine content are the main causes of the decreased weal and are reactions to codeine after clobetasol administration.
References
- Cohan VL, Undem BJ, Fox CC, Adhinson NF, Lichtenstein LM, et al. (1989) Dexamethasone does not inhibit the release of mediators from human mast cells residing in airway, intestine or skin. Am Rev Respir Dis 140: 951-954.
[Crossref], [Google Scholar], [Indexed]
- Schleimer RP, Schulman ES, MacGlashan DW, Peters SP, Hayes EC, et al. (1983). Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest 71: 1830 − 1835.
[Crossref], [Google Scholar],[Indexed]
- Atkins PC, Schwartz LB, Adhinson NF, Von Allmen C, Valenzano M et al. (1990) In vivo antigen-induced cutaneous mediator release: Simultaneous comparisons of histamine, tryptase, and prostaglandin D2 release and the effect of oral corticosteroid administration. J Allergy Clin Immunol 86: 360-370.
[Crossref], [Google Scholar], [Indexed]
- Noord HB, Orie NGM, Vries H (1971) Immediate and late bronchial obstructive reactions to inhalation of house dust and protective effects of disodium cromoglycate and prednisolone. J Allergy Clin Immunol 48: 344-354.
[Crossref], [Google Scholar], [Indexed]
- Slott RI, Zweiman B (1974) A controlled study of the effect of corticosteroids on immediate skin test reactivity. J Allergy Clin Immunol 54: 229-234.
[Crossref], [Google Scholar], [Indexed]
- Dunsky EH, Zweiman B, Fischler E, Levy DA (1979) Early effects of corticosteroids on basophils, leukocyte histamine, and tissue histamine. J Allergy Clin Immunol 63: 42б-432.
[Crossref], [Google Scholar], [Indexed]
- Olson R, Harpinh MH, Shelanshi S, Athins PC, Zweiman B (1990) Skin reactivity to codeine and histamine during prolonged corticosteroid therapy. J Alleugy Clin Immunol 86: 153-159.
[Crossref], [Google Scholar],[Indexed]
- Pipkorn U, Hammarlund A, Enerbach L (1989) Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy 19: 19−25.
[Crossref], [Google Scholar],[Indexed]
- Lavker RM, Schechter NM (1985) Cutaneous mast cell depletion results from topical corticosteroid usage. J Immunol 135: 23б8-2373.
[Crossref], [Google Scholar],[Indexed]
- Andersson M, Pipkorn U (1987) Inhibition of the dermal immediate allergic reaction through prolonged treatment with topical glucocorticosteroids. J Allergy Clin Immunol 79: 345-349.
[Crossref], [Google Scholar], [Indexed]
- Perretti M, Ahluwalia A (2000) The microcirculation and inflammation: Site of action for glucocorticoids. Micrococcal Tion 7: 147-1б1.
[Crossref], [Google Scholar],[Indexed]
- Singh G, Singh PH (1986) Tachyphylaxis to topical steroid measured by histamine-induced wheal suppression. Int J Deumatol 25: 324-32б.
[Crossref], [Google Scholar],[Indexed]
- Stahle M, Hagermark O (1984) Effects of topically applied clobetasol 17 propionate on histamine release in human skin. Acta Deum Venereal 64: 239-242.
[Crossref], [Google Scholar], [Indexed]
- Goldsmith P, Bunher C, Leslie T, Foreman J, Dowd PM (199б) The effect of topical steroid on the actions of vasoconstrictor and vasodilator peptides in human skin. Skin Pharmacol 9: 289-297.
[Crossref], [Google Scholar], [Indexed]
- López-Campos C, Rincón-Castañeda CB, Cano-Ríos P, Martínez-Ordaz VA , Velasco-Rodríguez VM (1998) Is the histamine skin test inhibited by prednisone? Auch Med Res 29: 63-65.
- Clough GF, Bennett AR, Church MK (1998) Effects of H1 antagonists on the cutaneous vascular response to histamine and bradykinin: A study using scanning laser Doppler imaging. Bu J Dermatol 138: 80б-814.
[Crossref], [Google Scholar], [Indexed]
- Clough GF (1999) Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo. J Physiol 516: 549-557.
[Crossref], [Google Scholar], [Indexed]
- Skov PS, Mosebech H, Norn S, Weehe B (1985) Sensitive glass microfiber based-histamine analysis for allergy testing in washed blood cells. Allergy 40: 213-218.
[Crossref], [Google Scholar], [Indexed]
- Walls AF, Bennett AR, Mcbride HM, Glennie MJ, Holgate ST, et al. (1989) Human mast cell tryptase: A biochemical marker for mast cell activation. Biochem Soc Trans 17: 728-729.
[Crossref], [Google Scholar]
- Huang X, Lu L, Gush RJ, Boggett DM (199б) A new fast high resolution laser Doppler imager for clinical and research use. In Proceedings of the 6th Nould Conguess fou Micuociuculation, Bologna: 115-119.
- Petersen LJ, Church MH, Shov PS (1997) Histamine is released in the weal but not the flare following challenge of human skin in vivo: A micro dialysis study. Clin Exp Alleugy 27: 284-295.
[Crossref], [Google Scholar],[Indexed]
- Church MK, Collier HO, James GW (1972) The inhibition by dexamethasone and disodium cromoglycate of anaphylactic bronchoconstriction in the rat. Bu J Pharmacol 46: 5б-б5.
[Crossref], [Google Scholar], [Indexed]
- Marquardt Dl, Wasserman SI (1983) Modulation of rat serosal mast-cell biochemistry by in vivo dexamethasone administration. J Immunol 131: 934-939.
[Crossref], [Google Scholar], [Indexed]
- Daeron M, Sterh AR, Hirata F, Ishizaha T (1982) Biochemical analysis of glucocorticoid-induced inhibition of IgE-mediated histamine release from mouse mast cells. J Immunol 129: 1212-1218.
[Crossref], [Google Scholar], [Indexed]
- Shewell J, Long DA (195б) A species difference with regard to the effect of cortisone acetate on the body weight, ц-globulin and circulating antitoxin levels. J Hyg Camb 54: 452-4б0.
[Crossref], [Google Scholar], [Indexed]
- Telford JM, West GB (1961) Some effects of corticosteroids on metabolism of histamine and 5-hydroxytryptamine in rat. Bu J Pharmacal 16: 360-368.
[Crossref], [Google Scholar], [Indexed]
- Castells MC, Friend DS, Bunnell CA, HU XZ, Hraus M, et al. (1996) The presence of membrane-bound stem-cell factor on highly immature nonmetachromatic mast-cells in the peripheral-blood of a patient with aggressive systemic macrocytosis. J Allergy Clin Immunol 98: 831-840.
[Crossref], [Google Scholar], [Indexed]
- Costa JJ, Demetri GD, Harrist TJ, Dvorah AM, Hayes DF, et al. (1996) Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J Exp Med 183: 2б81-2б8б.
[Crossref], [Google Scholar], [Indexed]
- Demitsu T, Hiyosawa T, Hahurai M, Murata S, Yaoita H (1999) Local injection of recombinant human stem cell factor promotes human skin mast cell survival and neurofibroma cell proliferation in the transplanted neurofibroma in nude mice. Auch Dermatol Res 291: 318-324.
[Crossref], [Google Scholar], [Indexed]
- Finotto S, Mehori YA, Metcalfe DD (1997) Glucocorticoids decrease tissue mast cell number by reducing the production of the c-kit ligand, stem cell factor, by resident cells: In vitro and in vivo evidence in murine systems. J Clin Invest 99: 1721-1728.
[Crossref], [Google Scholar], [Indexed]
- Barnes PJ, Larin M (1997) Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066-1071.
[Crossref], [Google Scholar], [Indexed]
- Yoshikawa H, Nahajima Y, Tasaha H (1999) Glucocorticoid suppresses autocrine survival of mast cells by inhibiting IL-4 production and ICAM-1 expression. J Immunol 162: 6162-6170.
[Crossref], [Google Scholar], [Indexed]
- Moy AB, Winter M, Hamath A, Blachwell H, Reyes G, et al. (2000) Histamine alters endothelial barrier function at cell-cell and cell-matrix sites. Am J Physiol Lung Cell Mol Physiol 278: 888-898.
[Crossref], [Google Scholar],[Indexed]
- Hossmann HA, Hurter T, Oschlies U (1983) The effect of dexamethasone on serum protein extravasation and edema development in experimental brain tumors of cat. Acta Neuropathol 60: 223-231.
[Crossref], [Google Scholar],[Indexed]
- Singh S, Gupta A, Pandey SS, SINGH G (199б) Tachyphylaxis to histamine-induced weal suppression by topical 0.05% clobetasol propionate in normal versus croton oil-induced dermatitis skin. Dermatology 193: 121-123.
[Crossref], [Google Scholar], [Indexed]
- Schwartz E, Mezich IA, Gendimenico GJ, Hligman LH (1994) In Vivo prevention of corticosteroid-induced skin atrophy by tretinoin in the hairless mouse is accompanied by modulation of collagen, glycosaminoglycans, and fibronectin. J Invest Deumatol 102: 241−24б
[Crossref], [Google Scholar],[Indexed]
- Haapasaari H, Rossi O, Risteli J, Oiharinen A (1998) Effects of long term inhaled corticosteroids on skin collagen synthesis and thickness in asthmatic patients. Euu Respir J 11: 139-143.
[Crossref], [Google Scholar], [Indexed]
- Barnes PJ (1998) Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin Sci 94: 557-572.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences